The idea of something lasting forever can be an unnerving thought, especially when little is known about it. This unease is common when people talk about per- and polyfluoroalkyl substances, or PFAS, which cover a large category of thousands of man-made chemicals. These chemicals can be found in virtually every facet of life: air, water, soil and food.
PFAS chemicals’ longevity is due to their bonds between carbon and fluorine molecules, one of the strongest chemical bonds possible, which makes removal and breakdown of PFAS very difficult.
Scientists have known about these manufactured chemicals since the 1940s, though it has only been in more recent years that the rest of the world has taken notice of PFAS and their potential risks to human health.
As PFAS become more widely discussed, scientists and policymakers are studying what can be done to remove the “forever” from forever chemicals.
Estimated reading time: 7 minutes
Scientists and agencies work to dismantle forever chemicals
More Information
- What are PFAS?, TWRI
- Our Current Understanding of the Human Health and Environmental Risks of PFAS, U.S. Environmental Protection Agency
- Texas A&M AgriLife develops new bioremediation material to clean up ‘forever chemicals,' Texas A&M AgriLife Today
- Pervasive Problem, txH2O
Want to get txH20 delivered right to your inbox? Click to subscribe.
A complicated environmental problem
PFAS have two classifications: short-chain and long-chain, depending on their chemical make-up. While there is a little overlap, short-chain PFAS are typically defined as having less than six to eight carbon atoms, while long-chain PFAS have more than six to eight carbons.
Long-chain compounds have different rates of solubility, transport and toxicity than short-chain compounds.
While it was previously thought that short-chain PFAS posed less of a human health risk than long-chain and moved through the body faster, Center for Disease Control and Prevention studies have shown that short-chain PFAS actually build up in the body more rapidly and stay there longer.
EPA’s proposal to establish a national standard for PFAS in drinking water is informed by the best available science and would help provide states with the guidance they need to make decisions that best protect their communities. This action has the potential to prevent tens of thousands of PFAS-related illnesses.
As more information becomes available about these forever chemicals, agencies have made new efforts to protect human and environmental health from PFAS impacts. In March of 2023, the U.S. Environmental Protection Agency (EPA) proposed a national standard for PFAS in drinking water.
“EPA’s proposal to establish a national standard for PFAS in drinking water is informed by the best available science and would help provide states with the guidance they need to make decisions that best protect their communities,” EPA Administrator Michael Regan said in a news release. “This action has the potential to prevent tens of thousands of PFAS-related illnesses.”
Such announcements have brought attention to the possible health impacts of long-term PFAS exposure. While research is still ongoing to understand how different types of PFAS and different levels of exposure can affect humans, EPA noted that there are possible health issues related to low-level, long-term exposure to harmful PFAS. Some of these possible issues, EPA said, include decreased fertility, an increased risk of some cancers, a reduced immune system, interference with natural hormones in the human body and potentially other unknown risks.
The true effects of PFAS are also hard to determine due to the sheer number of different PFAS, all with unique makeups and potential toxicities.
Testing PFAS removal technologies
Scientists like Xingmao ‘Samuel’ Ma, Ph.D., have taken a head-on approach to these questions. An associate professor in Texas A&M University’s Zachry Department of Civil and Environmental Engineering and at the Texas A&M Engineering Experiment Station (TEES), Ma currently has three funded projects tackling different angles of breaking down PFAS in the environment.
One of his research projects, funded through the Texas Water Resources Institute (TWRI) Faculty Fellows program, examines new ways to break down short-chain PFAS more effectively. Producing a single atom iron catalyst on a biochar support, Ma uses this carbon material to remove PFAS.
Through tests, Ma has found that while the reactions do not necessarily degrade or breakdown the PFAS alone, rapid adsorption occurs by the carbon iron material, which is typically the first step leading to eventual degradation of chemicals. Ma theorizes that iron’s positive electron charge might be one of the reasons why it works so well as a remover of negatively charged PFAS anions. He hopes to understand how other metals might work in the role too.
“The main question we’re trying to answer is whether incorporating metal atoms in a carbon support can generally improve PFAS removal and whether more redox active elements such as iron work better than other, more stable ones,” Ma said.
His second PFAS-related research effort is funded through the Water Exceptional Item, administered by TWRI, and looks to evaluate two light-sensitive photocatalysts. Ma is the principal investigator of the project, working with Anish Jantrania, Ph.D., and June Wolfe, Ph.D., Texas A&M AgriLife; Virender K. Sharma, Ph.D., Texas A&M School of Public Health; and Hongcai (Joe) Zhou, Ph.D., Texas A&M College of Arts and Sciences.
Using light exposure, Ma and his team have seen that their tests on certain PFAS produce hydrated electrons and prove effective for the removal or breakdown of them in smaller samples.
“Our first system that we’ve tested in a lab seems to work well in part,” Ma said. “We already published one paper, and we’re working on the second one, and an exciting part is we’re also moving towards field tests.”
While the tests will be small, he said, being in the field will be much bigger when compared to the 20-milliliter tubes they currently test on.
Ma’s third PFAS research effort is funded through the U.S. Army Corps of Engineers Engineer Research and Development Center (ERDC) and builds upon his other research, trying to lower the cost of PFAS removal. Ma also works with Sharma on this project.
His team hopes that by using different removal systems, they can test their efficiency and see which ones have the highest potential of being deployed in the field.
“We know the math works, but the model is expensive,” Ma said. “So, what we want to do is try a set of different systems and compare their efficiency for different types of PFAS to see which one really has the potential to work well in removal and also economically in natural water.”
Susie Dai, Ph.D., is another Texas A&M researcher studying the use of preexisting materials in the environment for PFAS removal.
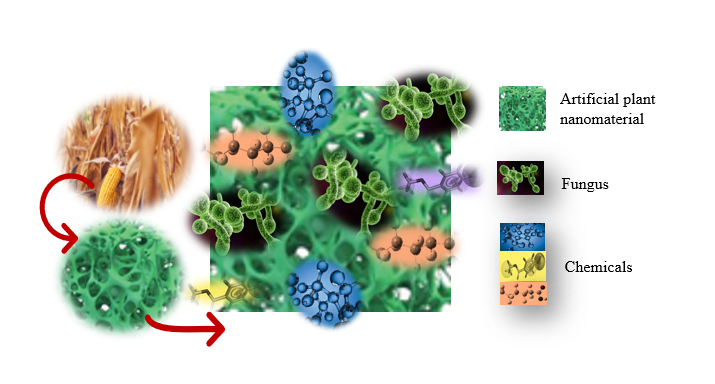
The fungus consumes plants. What I hope to do is use plant biomass to enrich the PFAS and then give that resulting stuff to the fungus to eat.
As an associate professor of plant pathology and microbiology in Texas A&M's College of Agriculture and Life Sciences, Dai and collaborators developed a technique of using a plant-derived material to adsorb the PFAS, and then disposing of them with microbial fungi that literally eat the forever chemicals.

“The fungus consumes plants,” Dai said. “What I hope to do is use plant biomass to enrich the PFAS and then give that resulting stuff to the fungus to eat.”
She explains that certain types of fungi consume lignin, complex polymers found in wood and bark that are resistant to breaking down, as their natural food. Understanding the interactions between fungi and PFAS and how they can be applied to PFAS removal is crucial, she said.
“Humans have different personalities, and fungi are the same,” she said. “There are so many different fungi in the environment. Some are more robust, some die easier, some like PFAs, some don’t.”
As the researchers continue to study and refine this remediation technique, Dai sees this method as a sustainable treatment system with the potential to remove harmful chemicals, protecting both human health and ecosystems in a non-toxic, cost-effective way.
Science informs policy
As researchers like Ma and Dai look to remove PFAS from the natural environment, government agencies want to educate the public about PFAS.
“Identifying the risk a chemical may pose to human health is a scientific process,” the EPA website reads. “There are likely thousands of PFAS that are currently present in the United States. Robust information about PFAS is needed to better understand the risks they pose and to be able to take effective actions.”
The U.S. Department of Defense (DoD) Strategic Environmental Research and Development Program (SERDP) also sponsors studies on PFAS treatment options. DoD used aqueous film-forming foam (AFFF) from the 1970s until production ceased in 2002.

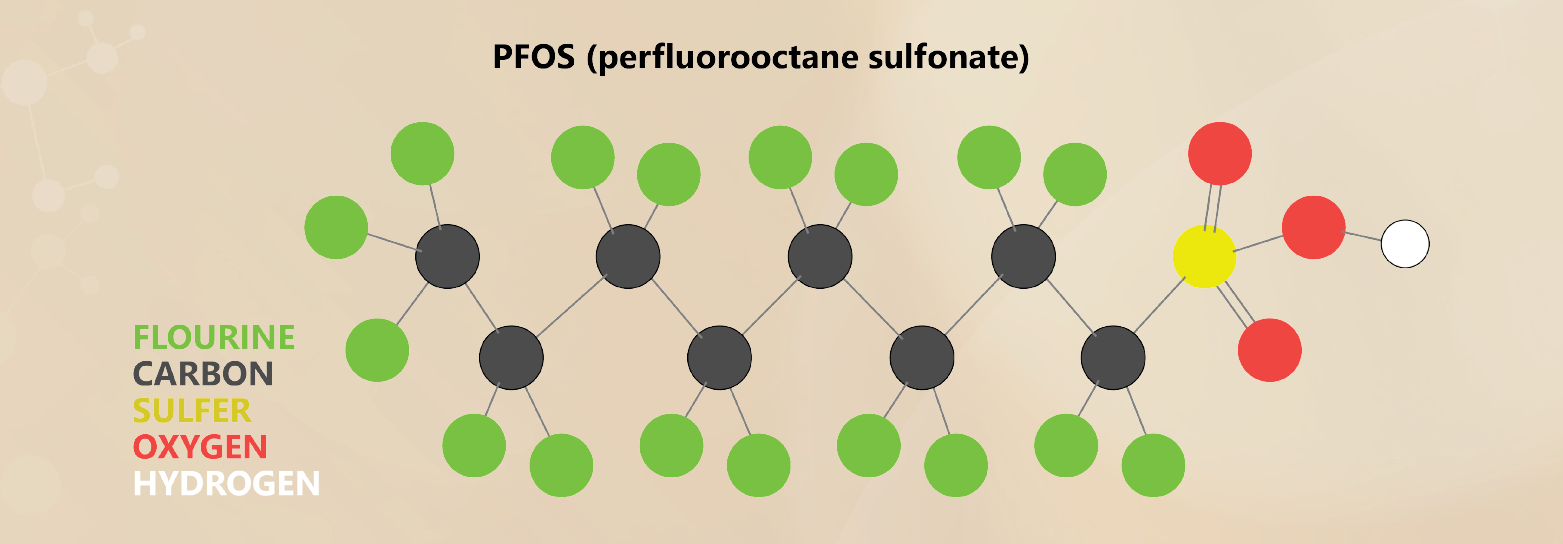
“The DoD used AFFF mixtures containing significant quantities of the PFAS perfluorooctane sulfonate (PFOS) and perfluoroalkyl sulfonates such as perfluorohexane sulfonate (PFHxS),” the SERDP website reads. “The potential magnitude of the DoD’s PFAS liabilities will require a sustained effort to identify the best technologies to characterize, treat and manage PFAS-impacted sites.”
AFFF was used for fire suppression in ships, shore-fixed systems and aircraft hangers, and to extinguish liquid fuel fires, according to DoD.
While the removal of chemicals called “forever” can seem daunting, research is showing that it is not impossible.
“Researchers and partners across the country are working hard to answer critical questions about PFAS,” the EPA website reads. “This information will help EPA and state, local and tribal partners make more informed decisions on how best to protect human health and the environment.”
As more studies are conducted to better understand and test the limits of PFAS, science will continue to reveal what researchers are up against.
Explore this Issue
Authors
Cameron Castilaw is a communication specialist at the Texas Water Resources Institute. She works with the communications team to create social media content, write for TWRI’s various platforms and print projects, and find new ways to inform people of TWRI’s mission and programs.