On Sunday, March 17, 2019, a fire started at the International Terminals Company (ITC) petrochemical plant in Deer Park, Texas. For four days, the fire burned, damaging 11 storage tanks and sending a huge plume of black smoke into the sky. High levels of the carcinogenic chemical benzene forced school closures and shelter-in-place orders in the Deer Park area.
On the third day, researchers led by Texas A&M School of Public Health research assistant professor Dr. Garett Sansom began collecting water, air and soil samples to understand the fire’s impact as part of an ongoing effort at the Texas A&M Superfund Research Center. In addition to benzene, they were looking for per- and polyfluoroalkyl substances, or PFAS, which would have resulted not from the fire, but from the firefighting foam used to put out the fire — and they found them.
Forever Chemicals
PFAS are a diverse group of more than 4,700 man-made chemicals defined by their bonds between carbon and fluorine molecules, one of the strongest known chemical bonds.
Those bonds make them so good at persisting in the environment and the human body that they’ve earned the nickname “forever chemicals.” PFAS are also highly heat resistant and repel both water and oil, which is why they are used in firefighting foam.
Dr. Kung-Hui “Bella” Chu, a professor in Texas A&M University’s Zachry Department of Civil and Environmental Engineering, said that PFAS are critical ingredients in aqueous film-forming foams used in fighting high-hazard flammable liquid fires.
PFAS are a diverse group of more than 4,700 man-made chemicals. Texas researchers are taking on the task of removing these long-lasting chemicals from the environment.
More Information
- How does EPA develop standards for contaminants?
- PFAS information from the EPA
- National Center for Electron Beam Research
Want to get txH20 delivered right to your inbox? Click to subscribe.
“PFAS act as surfactants to assist with putting out fire because they modify the surface tension of the flammable liquids quickly and cut off the oxygen, preventing reignition,” said Chu, who studies biodegradation and treatment of PFAS and other emerging contaminants.
PFAS were first introduced in the 1940s, when DuPont, an American chemical company, used a type of PFAS as an ingredient in the nonstick coating Teflon. Soon after DuPont invented Teflon, the 3M Company created Scotchgard, a stain- and water-repellant product for fabric, using a different kind of PFAS. By the 1960s, the Navy was working with 3M to develop PFAS-containing firefighting foam. PFAS have since found their way into a variety of household products, from food packaging to carpet.
The qualities that first made PFAS so useful in things like firefighting foam can also make them problematic, Sansom said.
“We have used PFAS in everything. They have certain really valuable chemical characteristics,” he said. “But we’ve been using them before fully understanding them. And now we’re playing catch up.”
PFAS’ strong chemical bonds protect them from degradation; the more carbons a PFAS chemical has, the better it is at persisting in the environment. PFAS can enter people’s systems via drinking water and some foods, and once in the body, PFAS’ ability to repel oil and water makes them difficult for the body to secrete, as they stick to fatty tissue.
“We have used PFAS in everything. They have certain really valuable chemical characteristics. But we’ve been using them before fully understanding them. And now we’re playing catch up.”
By the 1960s, both DuPont and the 3M Company expressed concerns about the toxicity of the PFAS they were using. In the late 1980s, the 3M Company found elevated cancer levels in PFAS workers. According to the U.S. Environmental Protection Agency (EPA), PFAS exposure is linked to a range of adverse health outcomes in humans. EPA also states that animal studies have shown that the two most well-known types of PFAS, perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), can cause reproductive, developmental, liver, kidney and immunological effects, as well as tumors. As people are exposed to more PFAS, they accumulate PFAS faster than their bodies can excrete them, in a process called bioaccumulation. Eventually, people reach a level of PFAS where they may experience adverse health effects. The exact level of exposure at which people begin experiencing the health effects is not yet known.
A little goes a long way
EPA recommends having no more than 70 parts per trillion of PFAS in drinking water. For comparison, one part per trillion is roughly equivalent to one drop of water in 20 Olympic-sized swimming pools of drinking water.
To express the smallness of the recommendation, Sansom compared it to the limit for lead, which is measured in parts per billion.
“The recommended maximum level of PFAS is an entire three orders of magnitude smaller than for lead. It doesn’t take much PFAS to be a problem,” he said.
All agree that PFAS are a problem, but how much of a problem is uncertain; thus more study is needed.
A 2018 draft report from the Department of Health and Human Services’ Agency for Toxic Substances and Disease Registry found that the recommended maximum level for PFAS of 70 parts per trillion should actually be 7 to 10 times smaller.

A 2016 study from the Harvard School of Public Health found that six million U.S. residents’ drinking water had confirmed levels of PFAS higher than EPA’s recommended maximum level. Other estimates suggest that the number of people with PFAS-contaminated drinking water could be up to 18 times higher. As of 2017, data from the U.S. Department of Defense (DOD) and Northeastern University’s Social Science Environmental Health Institute suggests that nearly 500,000 Texans live within a 3-mile radius of sites where groundwater is contaminated at levels 100 times EPA’s recommended maximum level of PFAS.
One of the principle ways PFAS enter the environment is via PFAS-containing firefighting foam, according to EPA.
After firefighting foam is used — most commonly on military bases and at commercial airports — it can seep into the ground or run off into nearby bodies of water, Sansom said. That is what happened during the ITC petrochemical fire when a protective barrier to the Houston Ship Channel failed.
PFAS can also enter the environment when rainwater percolates through PFAS-containing waste in landfills, leaching out the PFAS. That PFAS-containing water, called landfill leachate, either unintentionally seeps into the ground beneath the landfill or is pumped out of the landfill and released into the environment after being treated — but the traditional treatment methods don’t remove all the PFAS.
Industrial and domestic wastewater can also contain PFAS. In the wastewater treatment process, water is treated, and solids are concentrated. Because PFAS repel water, they tend to bind to solids over water, concentrating the PFAS in the solids. Those solids, called sewage sludge or biosolids, may be used as fertilizer after treatment, but again, that treatment doesn’t get rid of PFAS.
“With our good intentions to put biosolids to good use as land amendments, PFAS in biosolids have found their way into our environment and contribute to widespread PFAS contamination. And if you have some crop growing in biosolid-amended soils, the PFAS can accumulate in the crops and move up the food chain,” Chu said.
Treatment train
Tackling the pervasive problem of PFAS requires removing PFAS from the environment, regulating their use and replacing them with safer alternatives, Sansom said.
The effectiveness of different removal methods depends on the concentration of PFAS and the number of carbon bonds a particular type of PFAS has. PFAS with eight or more carbon bonds, called long-chain PFAS, are typically more toxic and accumulate more in humans and other animals than shorter chain PFAS.



Because different removal methods are helpful for different aspects of the PFAS problem, Chu said tackling PFAS removal requires a “treatment train” of multiple removal methods.
Many of the most common methods of PFAS removal use sorbents, or materials that can collect a liquid or gas, such as activated carbon. Water trickles through the activated carbon, which in turn takes up the PFAS, cleaning the water.
But, Chu said, sorbents such as activated carbon are an imperfect solution. While activated carbon and many other sorbents work well for long-chain PFAS, they typically don’t work equally well for short-chain PFAS, she said. After the sorbents are used on contaminated water, such as from landfill leachate or groundwater near heavy firefighting foam use, the PFAS-laden sorbents are considered hazardous materials. Neither incineration nor the current regeneration methods of the sorbents is cost-effective, she said.
Chu, along with a colleague at Texas A&M, has developed an alternative sorbent to activated carbon: reusable functionalized hydrogels. The hydrogels, which are “like jelly, but with stronger mechanical strength,” are reusable, decreasing treatment costs, Chu said. Most importantly, she said, the functionalized hydrogels can effectively remove short- and long-chain PFAS and GenX, a newer PFAS-containing replacement for PFOA, from water, putting them a step ahead of many other sorbents. Once the hydrogels have been used, the collected PFAS are removed from the hydrogels. The PFAS removed from the water must then be safely disposed of, usually by incineration, without re-releasing PFAS into the environment.
With our good intentions to put biosolids to good use as land amendments, PFAS in biosolids have found their way into our environment and contribute to widespread PFAS contamination. And if you have some crop growing in biosolid-amended soils, the PFAS can accumulate in the crops and move up the food chain.
Dr. Suresh Pillai, director of Texas A&M AgriLife Research’s National Center for Electron Beam Research at Texas A&M, is working on another step in the treatment train. He is researching the use of electron beam technology, or eBeam, to degrade PFAS in contaminated groundwater, wastewater, soil and biosolids.
eBeam technology works by accelerating electrons to 99.99% the speed of light and then showering the electrons over the material to be changed.
“eBeam packs a punch. It essentially shreds chemicals,” Pillai said.
The technology is already ubiquitous: It is used to colorize diamonds blue and green, sterilize contact lenses and disinfect mangos. When used on PFAS, Pillai said eBeam breaks the PFAS down into smaller byproducts. Research to confirm that those byproducts are completely harmless is ongoing.
The way eBeam works makes it flexible — it can be used either on untreated waste and water or on the PFAS removed with other methods, such as activated carbon or hydrogels.
“With eBeam, you can cover both the degradation and removal of PFAS, instead of using incineration. Moreover, eBeam can be used to degrade PFAS that were removed using other technologies, such as sorbents. So eBeam can either stand alone or work in conjunction with other technologies,” Pillai said.
Getting ahead of PFAS with replacements and regulations
Different industries use different types of PFAS, so replacing and regulating PFAS-containing products is not easy, Chu said.
Some major manufacturers of PFAS have phased out their use of long-chain PFAS, and the U.S. military is in the process of phasing out firefighting foam containing long-chain PFAS. Because PFAS’ useful qualities are chemically unique, most replacements are also PFAS — just shorter-chain PFAS, which are believed to be less bioaccumulative and easier for the body to secrete, Chu said.
“Anything that doesn’t stay in the body like long-chain PFAS do is a good thing,” she said.
But, Chu said, short-chain PFAS may cause the same health problems as long-chain PFAS, and they are more mobile than long-chain PFAS, meaning that they can travel farther in water and are therefore harder to catch and remove. It’s also unclear whether short-chain PFAS are any less environmentally persistent than long-chain PFAS, she said.
Research is being conducted on entirely PFAS-free firefighting foams. None have yet met DOD’s fire-extinguishing speed requirements; PFAS-free firefighting foam was 9 seconds slower than the requirement in U.S. Navy tests in 2004. However, some PFAS-free replacements have successfully been used to control jet fuel fires and match up with PFAS-containing firefighting foam in at least some scenarios. Since 2012, London’s Heathrow Airport has successfully switched to using PFAS-free firefighting foam, as have a number of other major European airports.
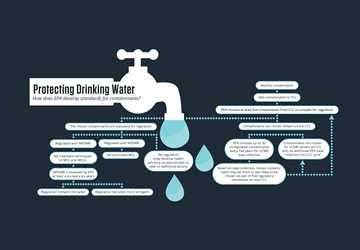
Though DOD has not settled on a firefighting foam replacement, regulations of PFAS may be upcoming. EPA’s current recommended maximum level of PFAS is a health advisory, not a regulation, and therefore is not legally enforceable. (For an explanation of how EPA makes drinking water regulations, see Protecting Drinking Water.)
In February 2020, EPA proposed preliminary regulatory determinations for PFOA and PFOS. According to the Federal Register notice submitted by EPA, the preliminary regulatory determination is the beginning of the process for developing regulations, not the end. The notice states that EPA may still decide later to not regulate PFOA and PFOS. In the meantime, at least nine states have developed their own PFAS standards. In December 2019, Congress finalized the National Defense Authorization Act (NDAA) for fiscal year 2020, which included some PFAS-related provisions, such as expanding PFAS monitoring and phasing out military use of PFAS in firefighting foam and food packaging. An earlier provision to designate PFAS as hazardous substances under the Superfund law, which would have required most PFAS-contaminated sites to be cleaned up, did not make it into the final NDAA. Work on other potential drinking water and groundwater regulations for certain kinds of PFAS is ongoing.
Winds of change
The sheer diversity and pervasiveness of PFAS is unprecedented, Chu said, and continuing and expanding research on PFAS in Texas is vital.
“If you don’t look for something, you probably can’t find it,” she said. “We might not see all the effects caused by PFAS right now, but for the next generation, we don’t know what the problems could be. However, if we are proactive on PFAS issues in terms of treatment and minimizing exposure, many adverse outcomes associated with PFAS contamination can be avoided or minimized.”

Pillai, for his part, is optimistic.
“Our data from past PFAS eBeam research is extremely promising,” he said. “My optimism comes because I know the capability of this technology. And people are starting to see the value of this research.”
In September 2019, EPA awarded more than $1.3 million to Texas universities for research on risks and management of PFAS, including Pillai’s research on eBeam technology. Texas Tech University environmental engineering assistant professor Dr. Jennifer Guelfo’s research on landfill leachate also received funding.
Following the ITC petrochemical plant fire, Sansom’s research team continued sampling for PFAS in the Houston Ship Channel. Immediately after the fire, PFAS levels were above EPA’s drinking water maximum recommended level of 70 parts per trillion. By midsummer, PFAS levels in the Houston Ship Channel decreased, indicating that the PFAS were washing out into Galveston Bay and the Gulf of Mexico. When the last samples were taken in August 2019, PFAS levels were still above the drinking water maximum recommended level — but the ship channel is not a drinking water source. Local well water that many people use for drinking water, as well as fish in the ship channel, have not yet been tested for PFAS.
Before the ITC petrochemical plant fire, the baseline levels of PFAS near the ITC petrochemical plant weren’t known, Sansom said. The silver lining of the fire, he said, is that now his team’s post-fire monitoring data can help increase understanding of PFAS in the area in the future, in case of another similar event.
“What are the long-term implications of PFAS in the environment? We don’t know yet. And are events like the ITC petrochemical fire going to happen again? Almost assuredly. It’s something that we’re going to be living with for the foreseeable future,” Sansom said. “But change is on the wind.”
Explore this Issue
Authors
As a communications specialist for Texas Water Resources Institute, Chantal Cough-Schulze worked with the institute’s communications team writing articles for and editing txH2O magazine and TWRI's news section, developing TWRI multimedia materials and editing reports and education and outreach materials. She also served as the managing editor for the Texas Water Journal.